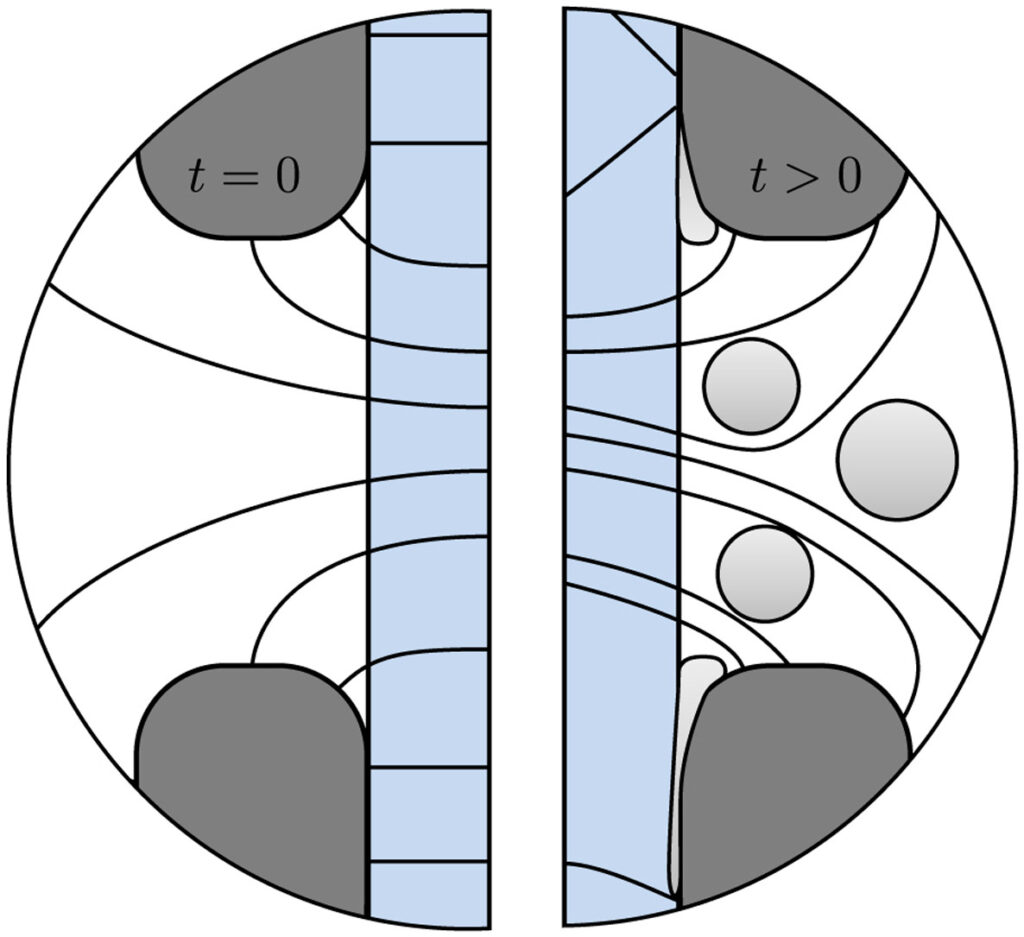
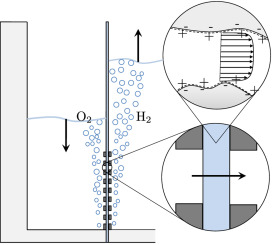
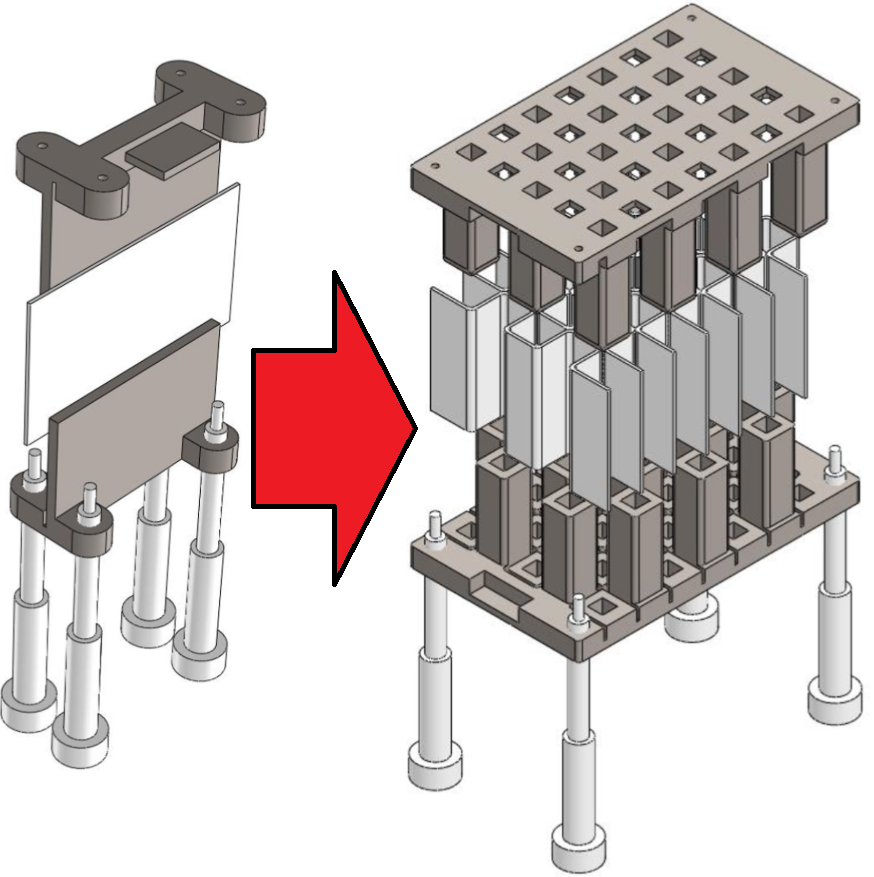
Water electrolyzers without a membrane have the potential to make green hydrogen more energy efficiently and cheaper. Perhaps the simplest type of membraneless electrolyzer consists of two vertical parallel plate electrodes with upwards electrolyte flow separating hydrogen and oxygen bubbles, avoiding the formation of an explosive mixture. The faster the flow, the thinner the bubble plumes and the closer the electrodes can be placed together, resulting in a higher energy efficiency. Natural convection can only provide modest velocities. Forced flow can provide higher velocities, but to avoid turbulent mixing of the bubble plumes, these are limited to similarly modest laminar flow velocities.
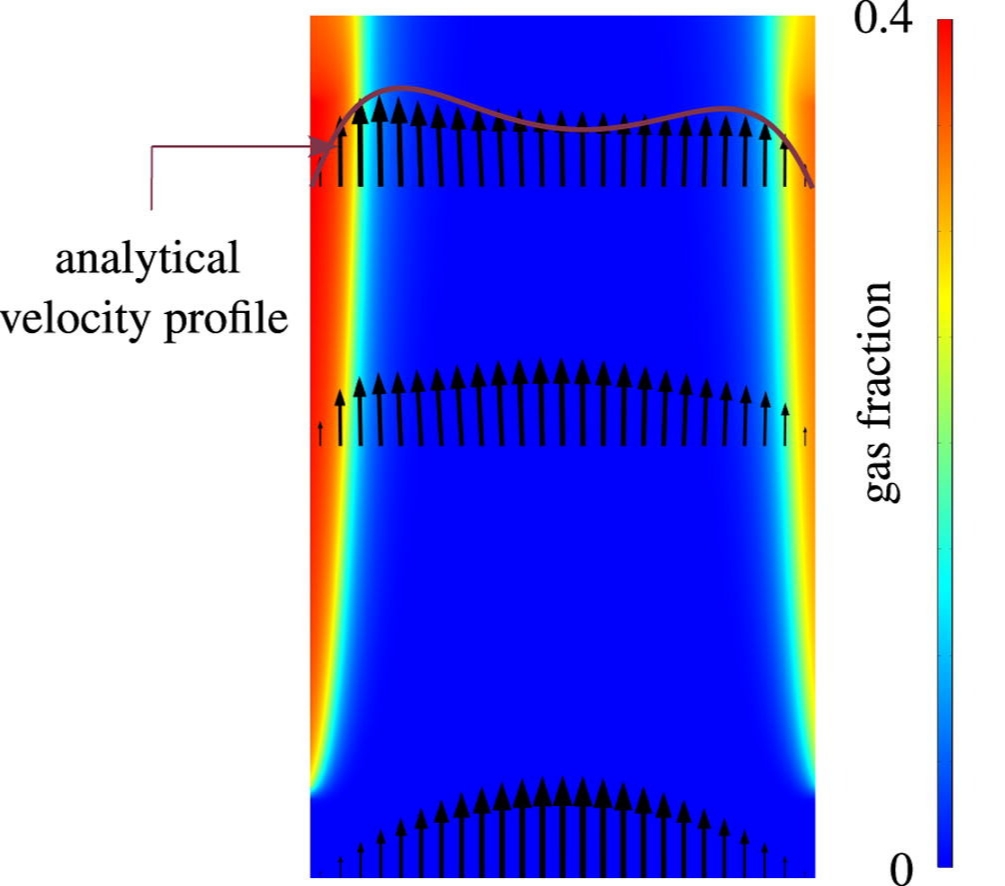
To determine how tall a membraneless electrolyzer can be made while still avoiding overlap between the oxygen and hydrogen gas plumes, we developed an analytical model that we verified with a more complete computational model and validated with experimental data from the literature. Based on our model we show that natural convection can allow safe and efficient atmospheric membraneless electrolysers up to about 5-10 cm height, while forced flow adds another 10 cm. At higher pressure, or by inducing smaller bubbles, taller or more energy efficient electrolysers of this type can be made.
See this previous post for an alternative type of membraneless electrolyzer.

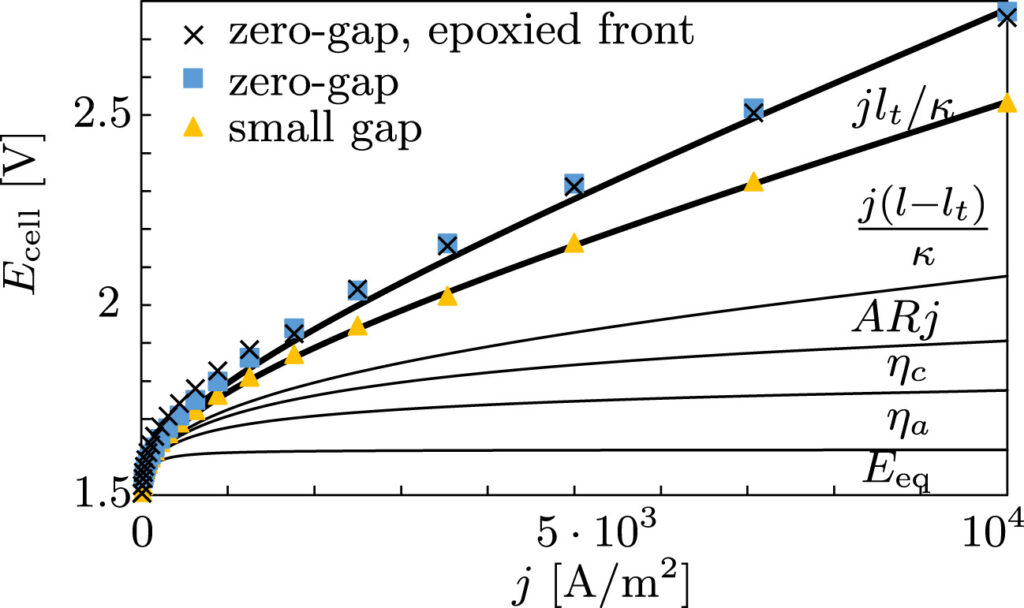
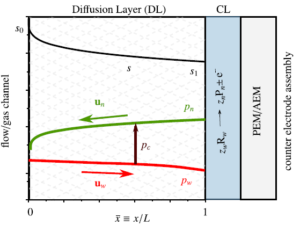
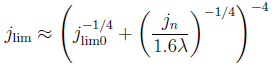
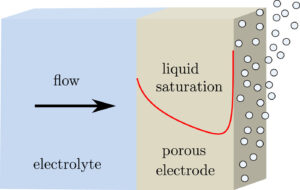
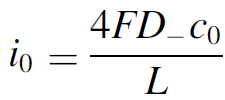
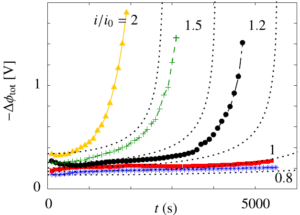
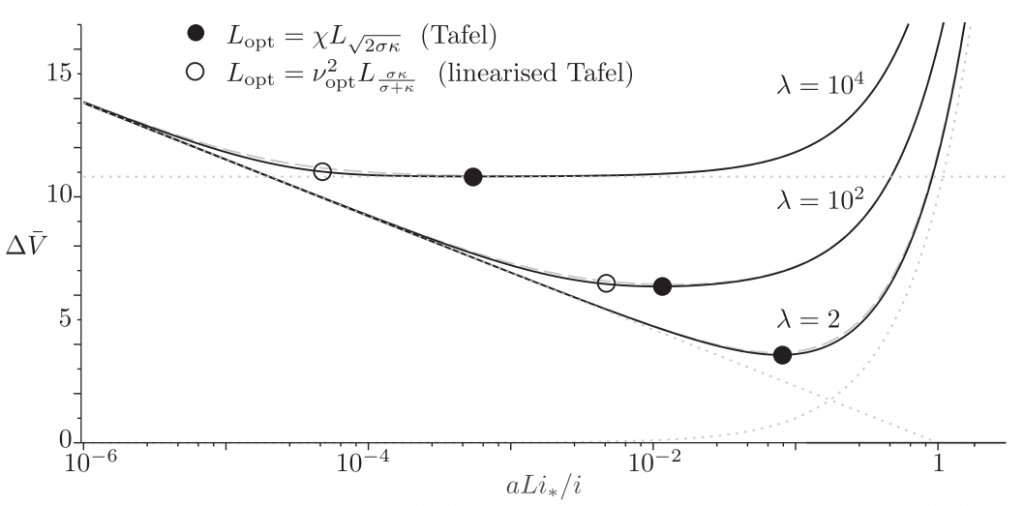 The dimensionless electrode overpotential versus electrode thickness. For the notation used see:
The dimensionless electrode overpotential versus electrode thickness. For the notation used see: