When making hydrogen in an electrolyser, at the same time also oxygen is produced. To avoid an explosive mixture, a sub-microporous separator is placed in between the electrodes. However, this separator leads to large energy losses and allows dissolved gas to pass (see also https://jwhaverkort.weblog.tudelft.nl/?p=140).
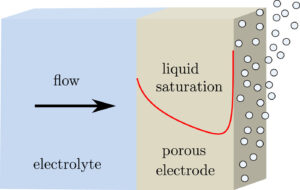
Electrolyte flow can be used to separate all hydrogen and oxygen and make for a membraneless electrolyser.
The question is whether flow can ensure separation more energy-efficiently than a physical separator. Using a combination of modelling and experiments we found the answer to be: yes!
Placing the electrodes approximately half a millimetre apart the ohmic losses can be made much smaller than with a separator, while the pumping power adds only a small additional loss.
