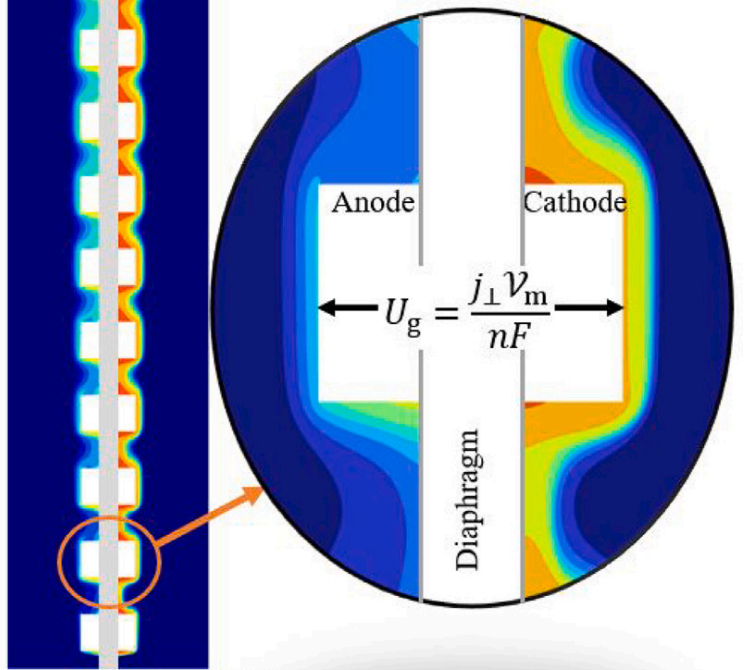
Electrodes in modern water electrolysers for the production of green hydrogen contain holes to let bubbles through, but what size should these holes be? Despite more than half a century since its first usage, there is no clear guidance on this question available in the open literature.
Therefore, we performed an extensive experimental campaign under industrially relevant conditions, testing 17 electrodes with varying hole sizes and shapes. The most important feature, by far, determining the electrode performance turned out to be the hole size, not its shape, the electrode thickness, or other features like the presence of pillars.
We found that for most relevant current densities and atmospheric pressure a hole size of several millimeters is optimal. Much larger holes lead to larger cell voltages as the current becomes more inhomogeneously distributed, increasing the resistance. But particularly, much smaller holes lead to excessive overpotentials.
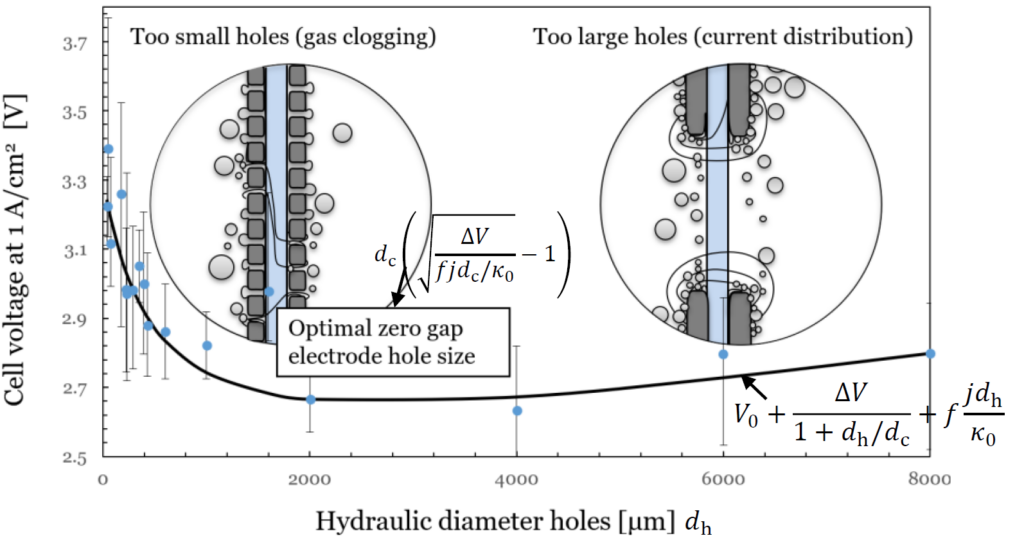
To study why this is, we looked at the behavior of gas bubbles, as they leave the holes from the back, but also from the front, through a transparent membrane. Holes small enough to be filled by a bubble equal to the hole size many times per second, tend to become clogged. As a result, a large gas film forms between the electrode and the diaphragm. This activates large parts of the electrode surface, forcing the reaction to the backside, increasing the resistance.
Millimeter-sized holes are largely immune to this clogging and perform much better, which explains why commercial expanded metal electrodes have holes in this size range.