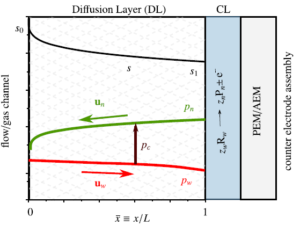
Many types of fuel cells and electrolyzers contain a diffusion layer, in between the reactant flow channels and the membrane. This porous layer protects the catalyst layer and provides an electronic connection to the current collector.
To allow reactants to reach the catalyst layer by diffusion, this layer should not be too thick. Liquid products can block the pores. We needed a somewhat lengthy and analysis to show that the resulting maximum current density reads
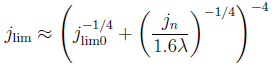
The first part describes the well-known diffusion-limited current density. The second term describes the reduction of the limiting current due to products blocking the pores. Here jn is a characteristic current density depending on e.g. reactant viscosity and surface tension, and λ is a parameter describingthe the pore size-distribution .
The effect of the second term in this equation is usually not dominant, decreasing the limiting current by less than half. Therefore, perhaps somewhat underwhelming, the result of our mathematical exercise is that modern diffusion layers are quite well-designed. At least we now know why and under what conditions.



