The electrochemical reduction of CO2 from flue gas, or even directly from the atmosphere, to useful products is an alluring prospect. Liquid fuels like gasoline can be made our of just air, water, and renewable electricity. One of the most promising routes is to reduce CO2 and H2O to CO and H2, or syngas. This can be done in a gas-diffusion electrode using silver catalyst particles.
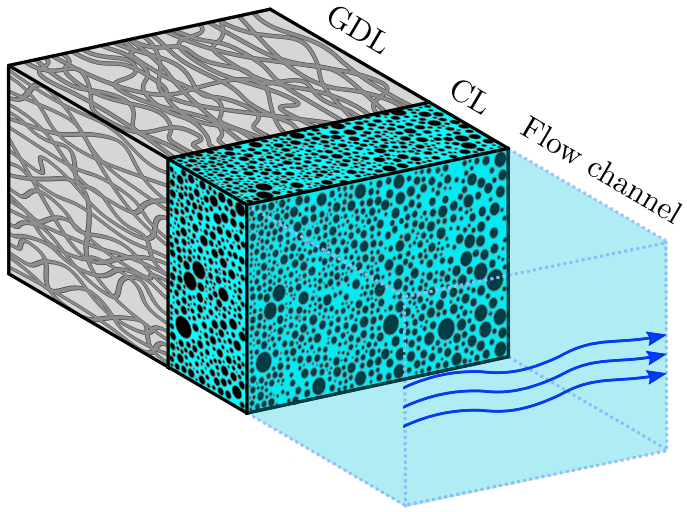
In a previous post we reported on a fully analytical multiphase model of the gas-diffusion layer (GDL). Recently, we succeeded in making an analytical model of the catalyst layer (CL), including transport, electrochemical reactions and homogeneous reactions involving CO2, OH–, HCO3–, and CO32-.
Our publication comes with a spreadsheet you can download that implements our analytical model.
