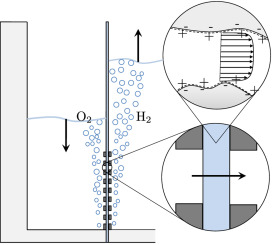
In a simple electrolysis experiment we found the cathode electrolyte level to decrease by several centimetre over the course of an hour, while that of the anode increased by a similar amount.
Since water is consumed at the cathode and produced at the anode and hydroxide ions drag still more water to the anode, this is an unexpected result. Our explanation is that the electric field, acting on positive charges near the separator pore walls, gives rise to an electro-osmotic flow from anode to cathode.
This flow impacts the limiting current as well as the crossover of dissolved hydrogen and oxygen and can therefore be useful in increasing the hydrogen purity and extending the operational range.
For more information, see:
and



