Mass transfer at electrodes with gaseous products is excellent, because bubbles that coalesce or leave the electrode mix the fluid very well. Additionally, rising bubbles set the electrolyte into motion causing additional flow transport. Traditionally, these two contributions, kμ and kf, have been simply added. To improve upon this oversimplification, a new simple ‘addition rule’ was devised

which takes into account the surface coverage θ of bubbles and a single empirical micromixing parameter a.
Next, this formula was compared with all available literature data for water electrolysis, revealing large differences. For hydrogen in alkaline electrolytes, bubbles hardly mix and the mass transfer is determined by natural convection. For hydrogen bubbles in sulphuric acid, on the other hand, natural convection can be usually neglected and for other cases, both mechanisms can be important.
The reason why there are these large differences in the parameter a is hypothesized to be due to solutal Marangoni convection, which repels hydrogen bubbles away from each other and the electrode in alkaline electrolytes while it attracts hydrogen bubbles towards each other and the electrodes in sulphuric acid.
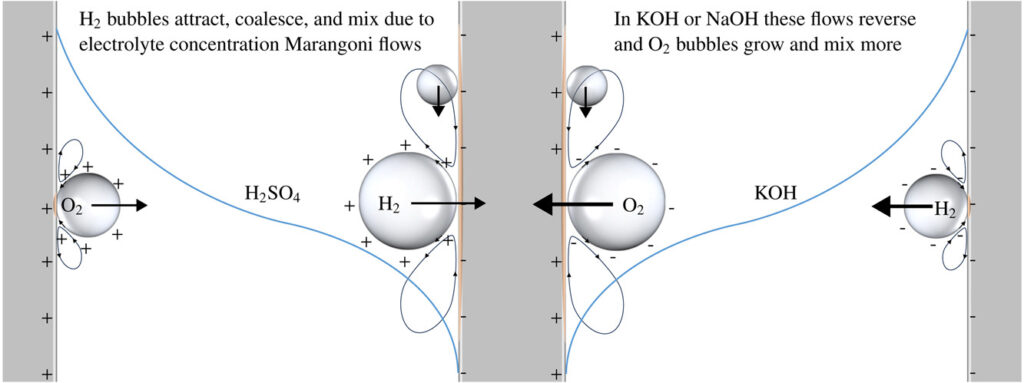
Haverkort, J. W. (2024). A general mass transfer equation for gas-evolving electrodes Int. J. Hydrogen Energy, 74, 283-296.



