Since water is the reactant in water electrolysis, you may be excused for thinking there will be no diffusion limitations. However, at the anode of an alkaline water electrolyzer the reactant is hydroxide (OH-), produced at the cathode. Although usually present at very high concentrations c0 of 6 or 7 M, these ions may deplete at the anode, leading to a limiting current density given by:
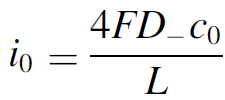
With a typical separator thickness L of 0.5 mm and effective diffusivity D– of 10-9 m2/s this gives about 0.5 A/cm2, in the operating range of modern electrolyzers.
In the following graph, the measured voltage over the separator can be seen to diverge when a current larger than i0 is applied. The dashed lines show the behavior expected from a simple model.
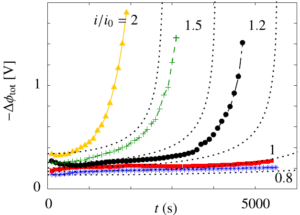
For more information on the these measurements, the model, and their relevance for hydrogen production see:
and



