In alkaline water electrolysers for hydrogen production, mesh electrodes are separated by a porous separator to avoid mixing of hydrogen and oxygen bubbles. Since the ohmic resistance increases with the distance over which the current travels, you would expect that minimizing the distance between the electrodes leads to the highest energy efficiency. Therefore, the so-called zero-gap configuration, in which the electrodes are placed directly adjacent to the separator, has become the standard in industrial electrolysers.
However, it has been found, time and again, that the ohmic resistance of zero-gap configurations is substantially higher than expected from the separator resistance. This can be partially attributed to the current lines not being straight in a zero-gap configuration, see the below figure. With detailed measurements we find that over the course of approximately 10 seconds the ohmic drop further increases. This can be attributed to the formation of gas bubbles, see the below figure. With this, we can now finally explain the anomalously high ohmic drops found in zero-gap water electrolysers.
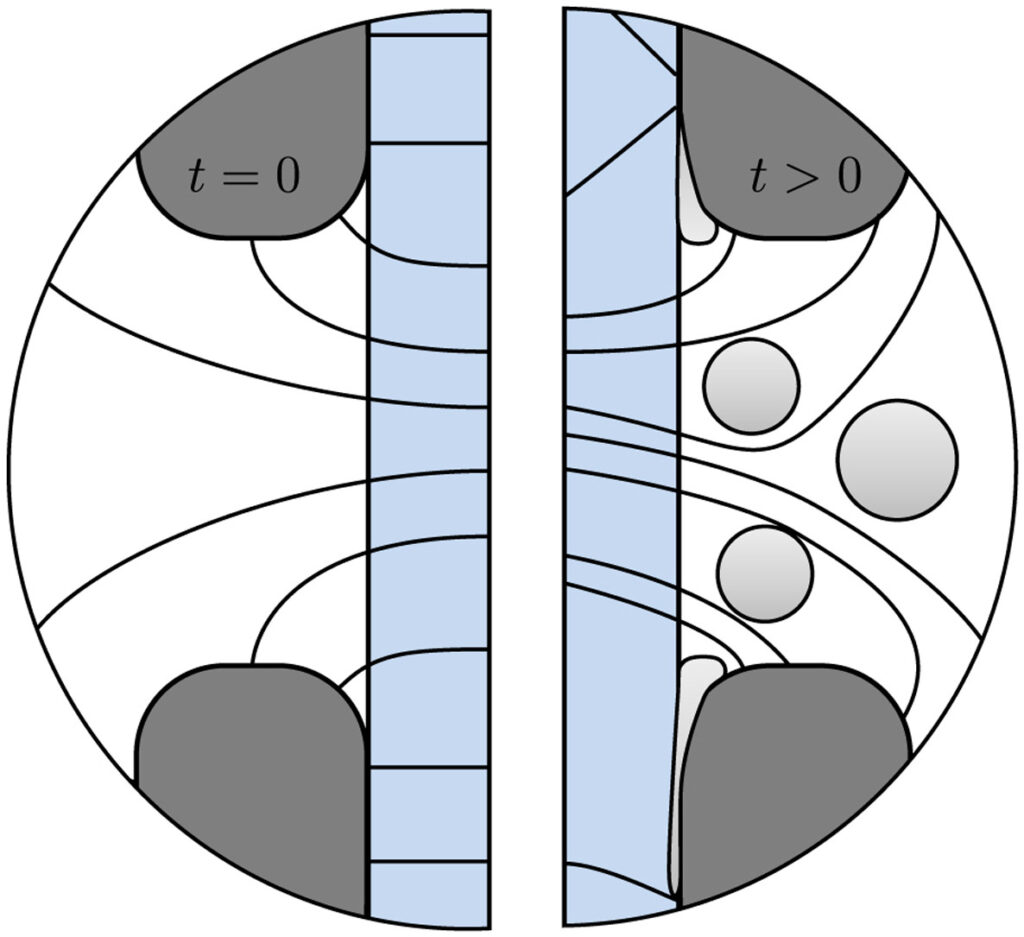
Besides these bubble-induced losses, our detailed measurements further quantify the often-overlooked effect of dissolved gases on the equilibrium potential (Eeq in the below figure) as well as the concentration overpotential due to hydroxide depletion, only relevant at low electrolyte concentrations.
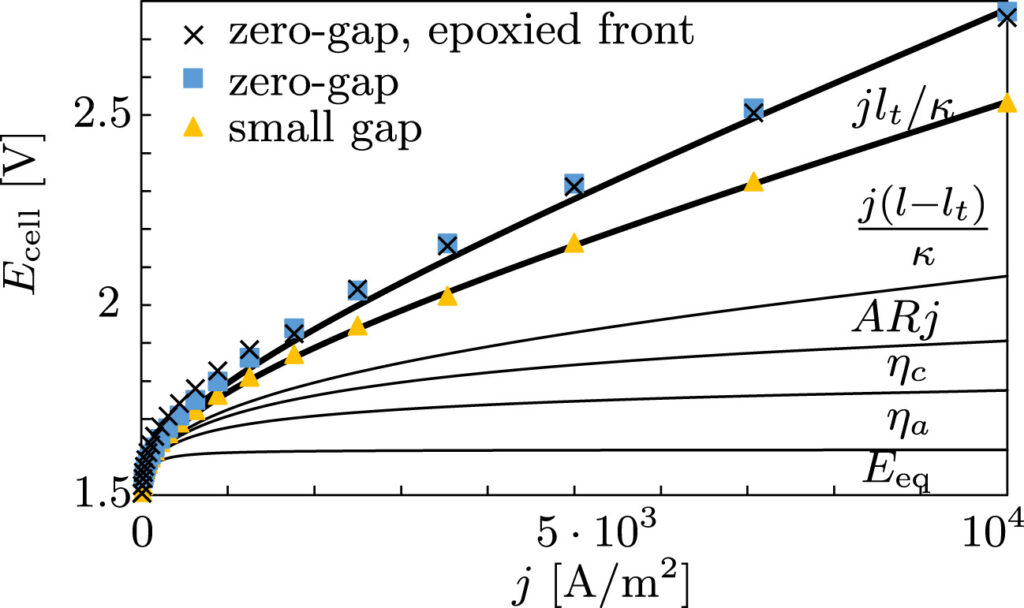
We also show that with a very small gap the ohmic drop can be significantly reduced, see the above figure. This initially counter-intuitive result can now be understood by the gap being large enough for bubbles to escape, allowing the current lines to become straighter. This simple and cheap way of increasing the efficiency of hydrogen production likely also leads to improved durability of the electrodes and separators as well as reduced gas cross-over. This can increase the turndown ratio, important for flexible operation of electrolysers with renewable energy.



