CO2-electrolysis is a promising way of making carbon-based fuels and chemicals from CO2, instead of from fossil fuels. Affordable electrolyzers that can efficiently convert CO2 and water into for example syngas (CO and H2) hold great promise for transforming the way carbon-based products can be industrially made in the future. There has been enormous research progress over the last few years, typically in lab set-ups with dimensions of a few centimeter. In a recent publication we tried to answer the question what would happen in a much taller electrolyzer. We find that much more of the CO2 entering the electrolyser is converted and also the fraction of the desirable CO in the outlet stream is increased. Unfortunately, our simulations show that this seemingly positive effect arises because more CO2 undergoes undesirable side-reactions with the electrolyte. As a partial remediation, we show that by varying the amount of catalyst along the electrolyzer this problem can be substantially reduced.
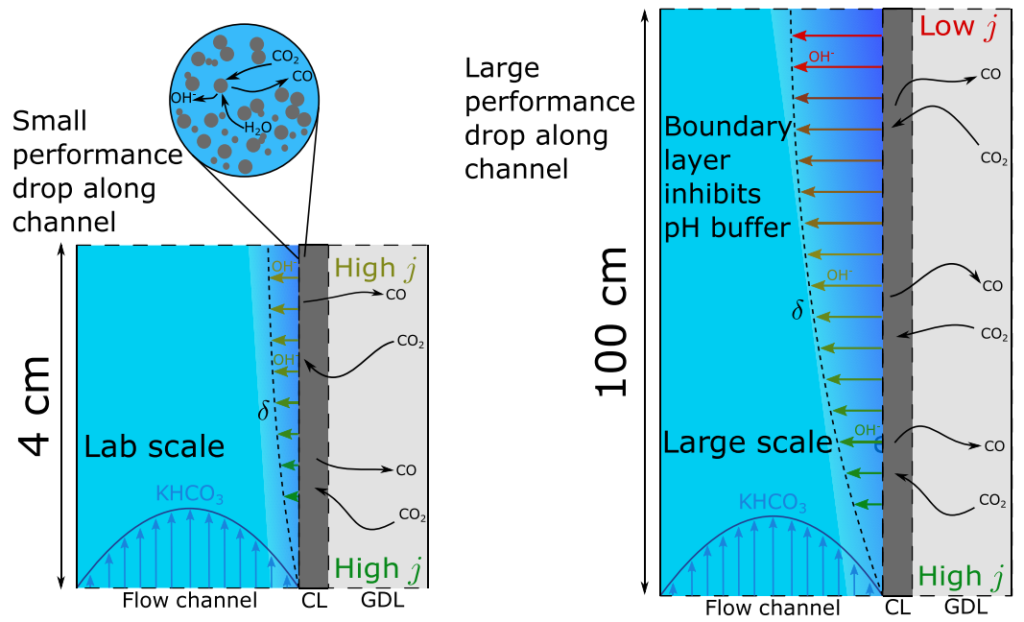
Joseph W. Blake, Vojtecȟ Konderla, Lorenz M. Baumgartner, David A. Vermaas, Johan T. Padding, and J. W. Haverkort, Inhomogeneities in the Catholyte Channel Limit the Upscaling of CO2 Flow Electrolysers, ACS Sustainable Chem. Eng. 2023, 11, 7, 2840–2852
