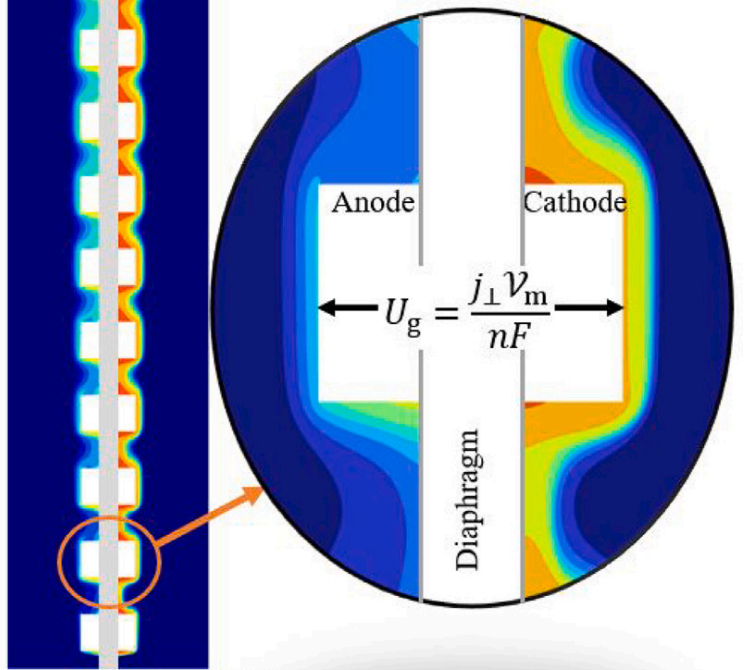
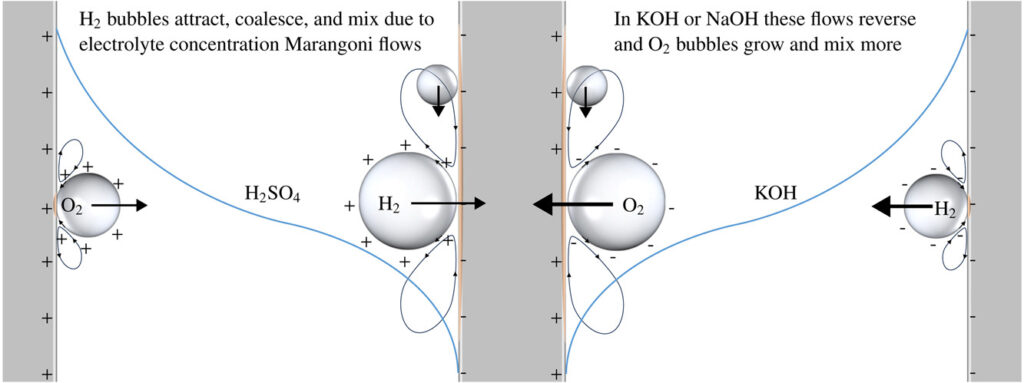
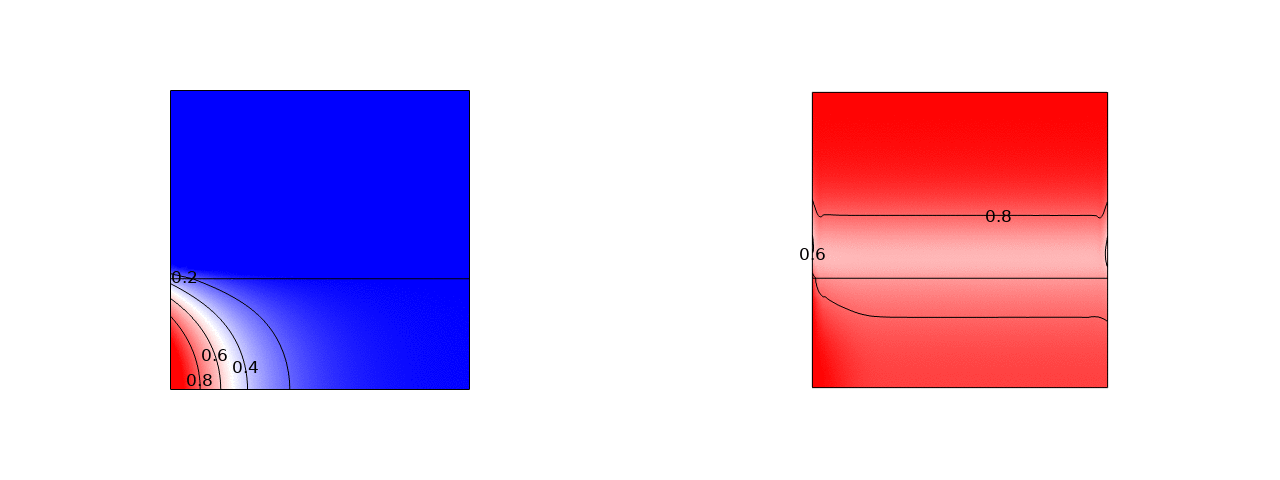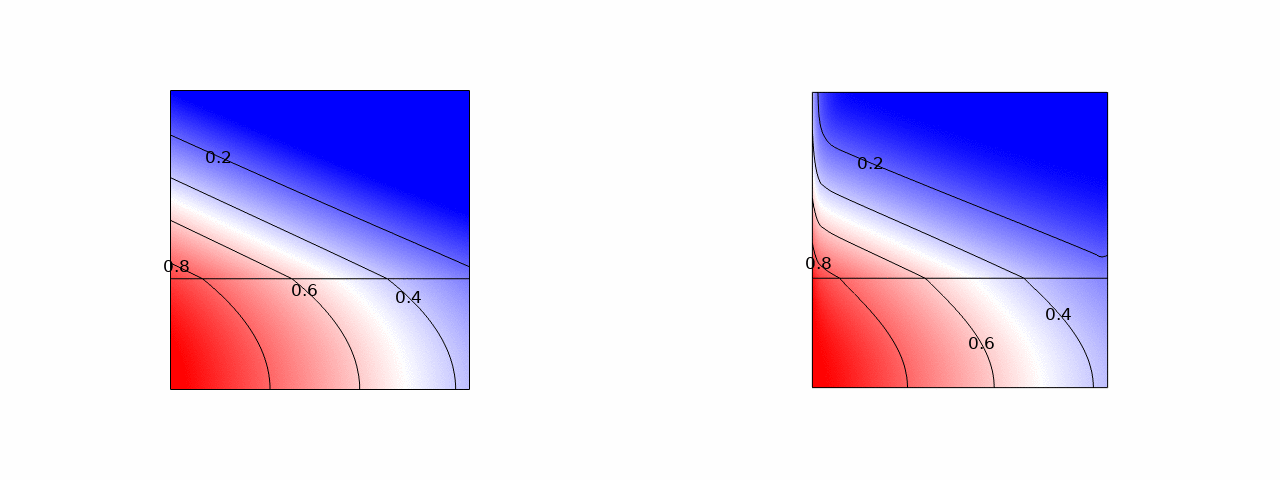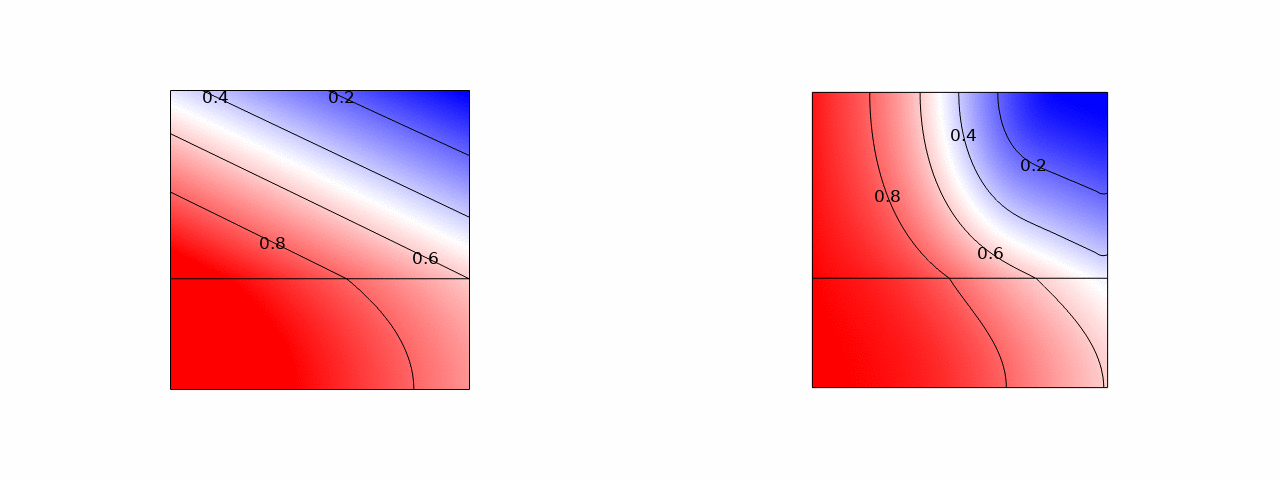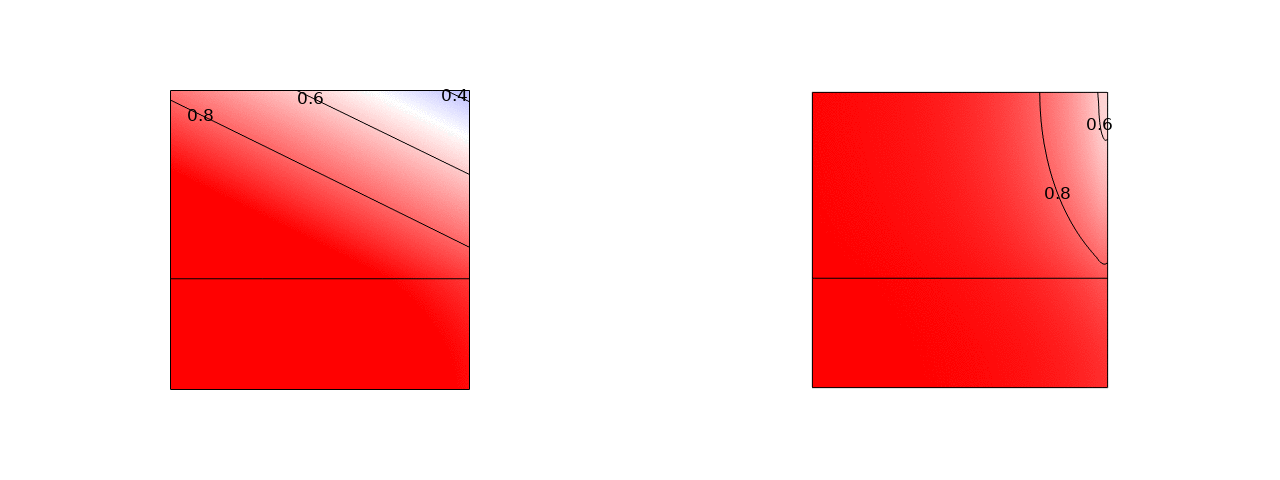
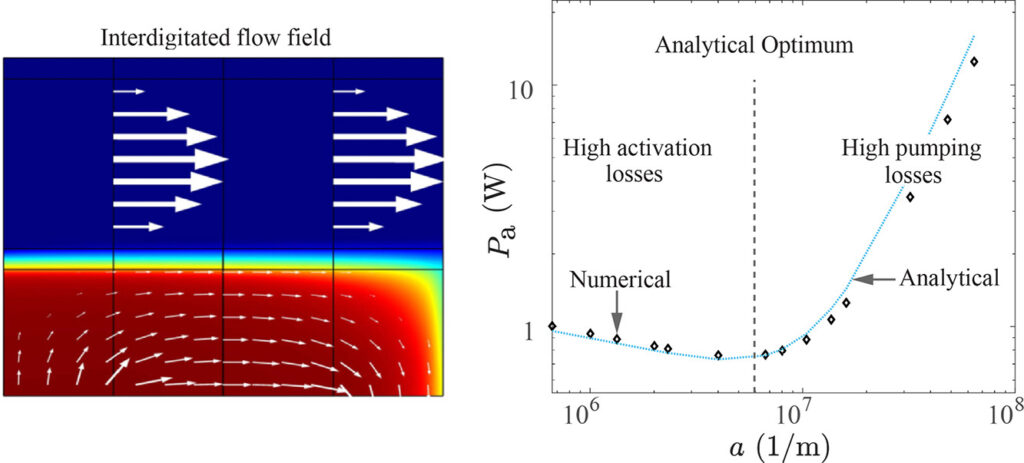
In stacks of electrolysers, redox flow batteries, or fuel cells with liquid electrolytes, ions can leave their half-cell through the fluid inlet or outlet ports and enter an entirely different half-cell. These pathways have a much higher resistance than the intended route through the membrane. However, in bipolar stacks, the driving force, the potential difference between half-cells that are far apart, is much larger. These ‘shunt currents’ can deteriorate the efficiency of electrolysers or drain a flow battery through internal self-discharge.

Therefore, many scientific works have measured shunt currents and successfully modelled them by numerically solving an equivalent circuit. It turns out that a very simple and intuitive approximation is also possible. The average shunt current in the manifold can be approximated by the average potential difference between cells divided by the series resistance of the manifold Rmn and the inlet/outlet port resistance Rio. However, the latter should be multiplied by 12/N(N+1), where N is the total number of cells in the stack. This can be shown both from a limiting-case solution, possibly dating back to a 1930s Russian book, and from correcting an overlooked German-language exact solution.