Various electrochemical cells including microfluidic fuel cells, membraneless redox flow batteries, and microfluidic (CO2) electrolysers include a channel between their two electrodes. The wider this channel, the larger the ohmic drop. The thinner this channel, the larger the pressure drop. We obtained a simple analytical formula for the optimal channel width that minimizes the combined associated energy dissipation, which we verified using numerical simulations.
To increase surface area while avoiding diffusion limitations, sometimes porous flow-through electrodes are used. Here, a similar optimization can be performed. Smaller pore sizes give more reactive surface area, but also a larger pressure drop. Also here an analytical formula was obtained, which works well even for the popular interdigitated flow configuration.
We compared our formulas with values from various papers in the literature and found that typically an order of magnitude too large channels and pores are used. Therefore, significant improvements in energy efficiency can be obtained by further miniaturization.
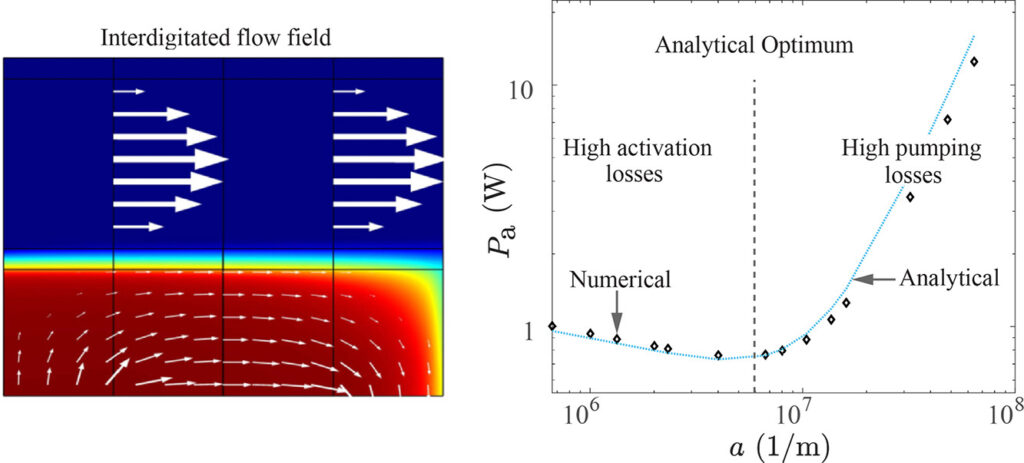
The flow velocity (arrows) and reactant concentration (color) inside a repeating unit of an interdigitated flow field (left). The combined activation and pumping dissipation from simulations (diamonds) and the obtained analytical expression (solid line) along with the optimal (dashed vertical line) volumetric surface area a (roughly the inverse of pore size).



