Most electrodes used in commercial applications like batteries and fuel cells contain electrodes that are porous to increase the reactive surface area. Strangely, a hitherto largely unanswered but important question is how thick such electrodes should ideally be. Thick electrodes will gives a high resistance while thin electrodes less surface area. Unsurprisingly there is an optimum, graphically shown in the below graph.
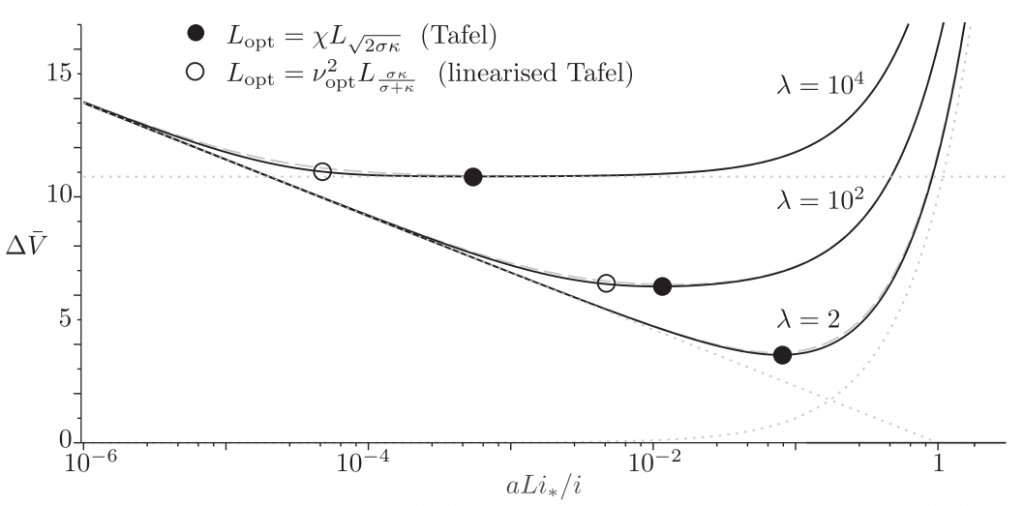 The dimensionless electrode overpotential versus electrode thickness. For the notation used see: https://doi.org/10.1016/j.electacta.2018.10.065
The dimensionless electrode overpotential versus electrode thickness. For the notation used see: https://doi.org/10.1016/j.electacta.2018.10.065
The seminal 1962 paper of Newman and Tobias provided exact but implicit analytical solutions. Introducing a generalization of the effectiveness factor concept, I obtained approximate explicit current-potential relations that are insightful and easy to use. Using these, analytical expressions could be derived for both the optimal electrode thickness and porosity of catalyst layers as well as battery electrodes.
“A theoretical analysis of the optimal electrode thickness and porosity” can be freely accessed through: https://doi.org/10.1016/j.electacta.2018.10.065
A poster summarizing the paper presented at Modval 2019 : poster
A short presentation adapted from a talk at the ISE 2018 conference in Bologna or at the ECCM Conference in The Hague in 2019.
An excellent first introduction to the modeling porous electrodes can be found at:http://www.joshuagallaway.com/?p=215



