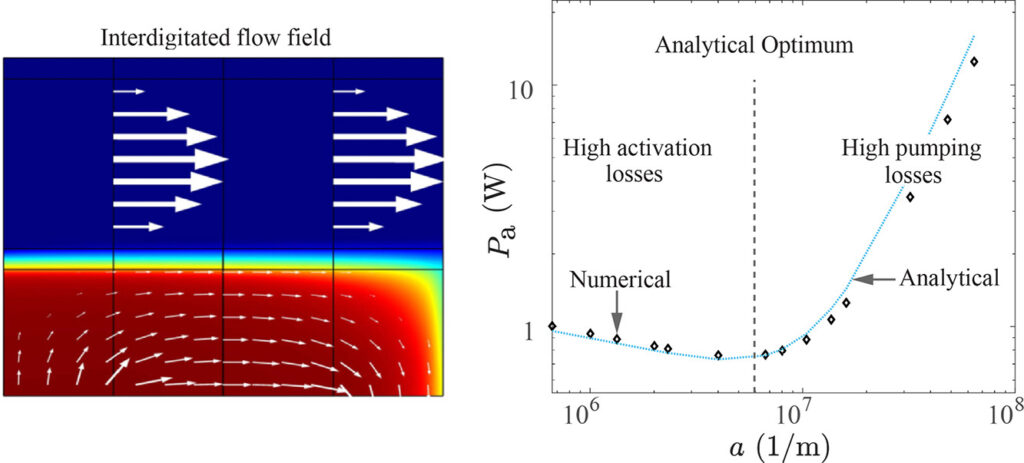
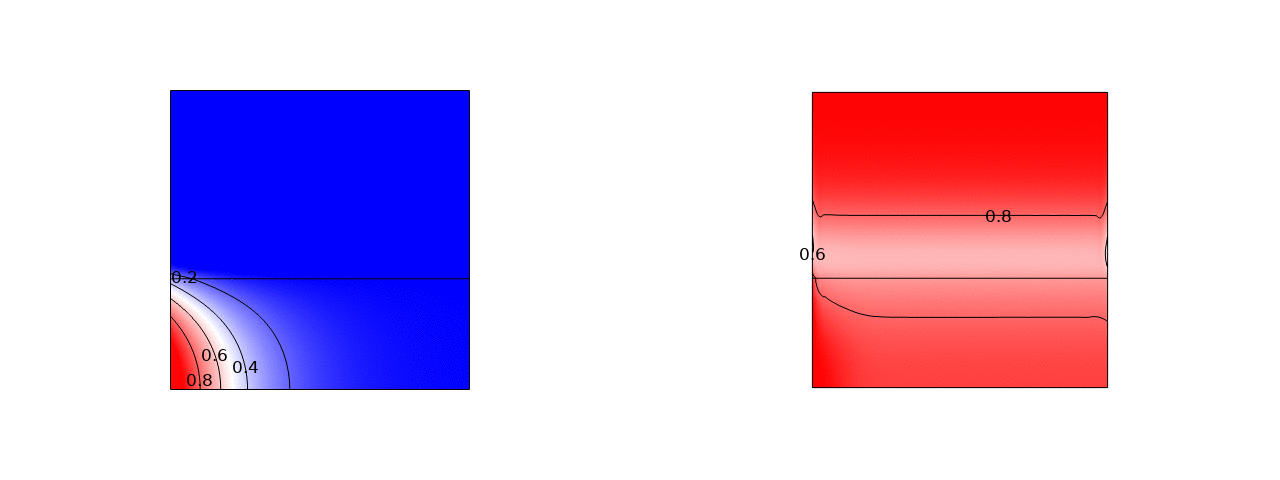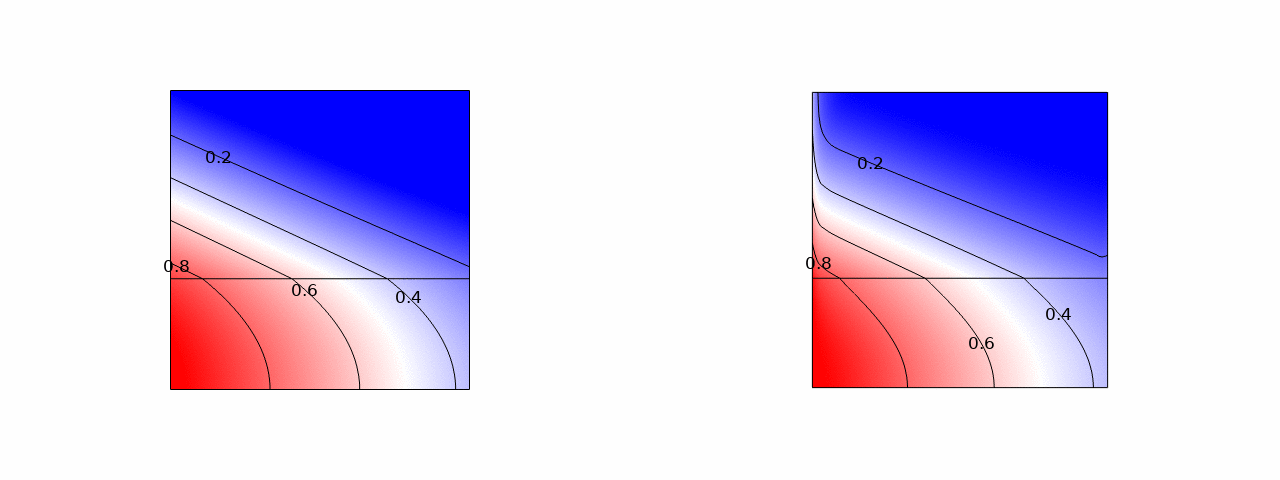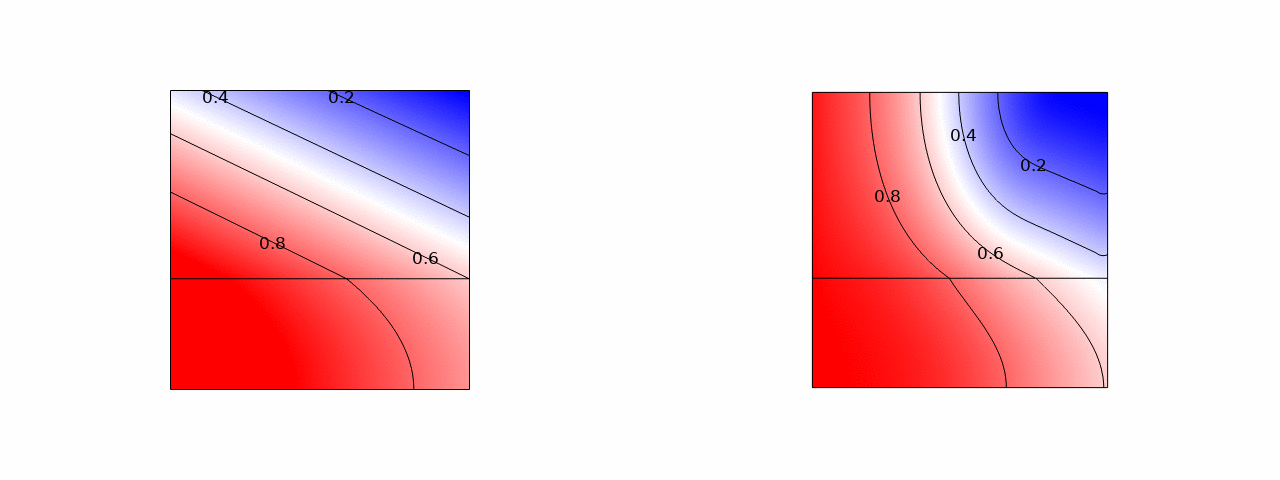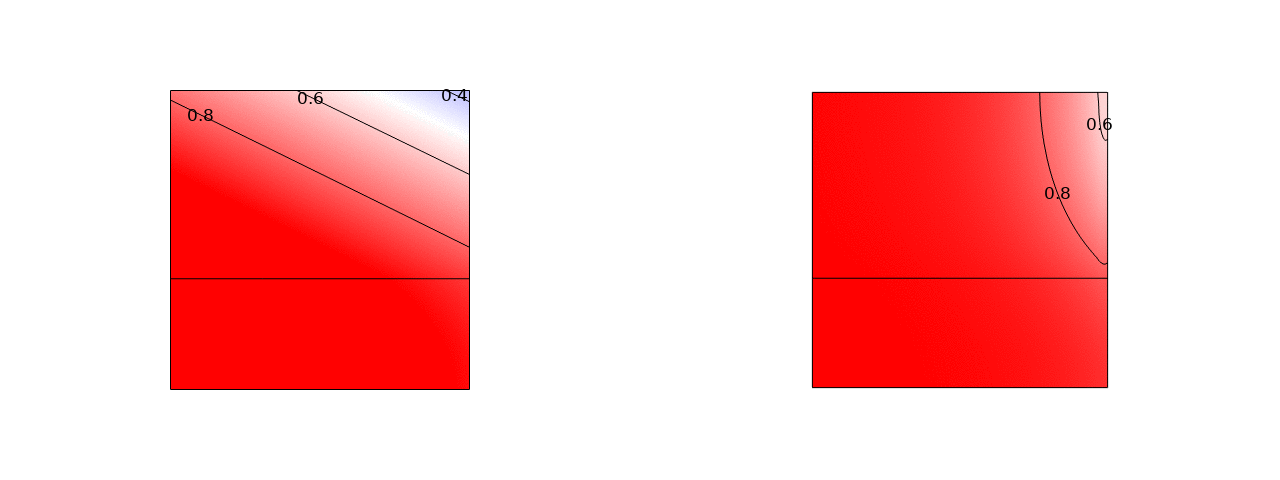
A promising way to remove low salt concentrations from brackish water is capacitive deionization (CDI). Instead of removing the water from the salt, as for example in reverse osmosis, in CDI the salt ions are removed from the water by applying an electric field. The ions are removed from the main flow and stored in the electric double layers of the porous electrodes flanking the main flow channel. Despite the fact that this is an inherently two-dimensional and transient process, usually modelled with several partial differential equations, we managed to simplify the problem to two coupled ordinary differential equations and obtained an explicit analytical solution. Here is a comparison of the salt concentration with a comprehensive computational COMSOL model:
The power of such an analytical solution lies in making optimization much easier. Instead of seven individual physical and geometrical parameters, time, and a spatial coordinate, our solution primarily depends on a single dimensionless number that is a combination of these parameters.
We find that the optimal porous electrodes are roughly six times thinner than the spacer. By minimizing the energy losses and maximizing the amount of water processed, we find that an optimal design can increase the latter metric by an order of magnitude compared to typical values in the literature.
J.W. Haverkort, B. Sanderse, J.T. Padding, and J.W. Blake (2024). An analytical
ow-by capacitive deionization mode. Desalination.